Science GCSE
PHYSICS
Energy and the future
Energy transfer and
efficiency
energy: The ability to do work or cause movement.
There
are several different types of energy which can be transferred from one type to
another. Energy transfer diagrams show these transfers in process. More
efficient devices transfer the energy supplied to them into a greater
proportion of useful energy.
Forms of energy
You
should be able to recognise the main types of energy. Mainly there are 9
different types of energy. One way to remember the different types of energy is
to learn this mnemonic sentence:
Most Kids Hate Learning GCSE Energy Names
where each
capital letter is the first letter in the name of a type of energy.
Types of
energy/Description/Example
- Magnetic: Energy in magnets and electromagnets. Example: Magnet
- Kinetic: The energy in moving objects. Also called movement energy. Example: A bullet cutting a playing card.
- Heat: Also called thermal energy. Example: Burning match
- Light: Also called radiant energy. Example: Sunlight
- Gravitational potential: Stored energy in raised objects. Example: Skydivers
- Chemical: Stored energy in fuel, foods and batteries. Example: Organic food
- Sound: Energy released by vibrating objects. Example: Guitar
- Electrical: Energy in moving or static electric charges. Example: Lightning
- Elastic potential: Stored energy in stretched or squashed objects. Example: Catapult
- Nuclear: Stored in the nuclei of atoms. Example: Nuclear fuel assembly
Energy transfers
In Key Stage 3 it is taught how to draw
and interpret an energy transfer diagram.
We will revisit energy transfer diagrams as follows:
We will revisit energy transfer diagrams as follows:
Different types of energy can be transferred from one type to
another.
Energy transfer diagrams show each type of energy, whether it is stored or not, and the
processes taking place as it is transferred.
Sankey diagram generic
Sankey diagram for a filament lamp
In the Sankey diagram for a filament lamp above one can see the relative amounts of each type of energy. The electrical energy is 100J. While the useful light energy given is 10J, the waste energy is the heat energy which is 90J.
Energy transfer diagrams
The following three energy transfer diagrams show the useful energy transfer in
a car engine. You can see that a car engine transfers chemical energy, which is
stored in the fuel, into kinetic energy in the engine and
wheels.
Diagram 2: Energy transfer diagram in a Car Engine
Diagram 3: Sankey diagram in a Car Engine
Diagram 1: Energy transfer diagram in a Car Engine
Diagram 2: Energy transfer diagram in a Car Engine
Diagram 3: Sankey diagram in a Car Engine
Process of using chemical energy
This diagram shows the energy transfer diagram for the useful
energy transfer in an electric lamp. You can see that the electric lamp
transfers or converts electrical energy into light
energy.
Process
of using electrical energy
Notice that
these energy transfer diagrams only show the useful energy transfers. However,
car engines are also noisy and hot, and electric lamps also give out heat
energy.
Sankey diagrams
Sankey
diagrams summarise all the energy transfers taking place in a process. The
thicker the line or arrow, the greater the amount of energy involved. The
Sankey diagram for an electric lamp below shows that most of the electrical
energy is transferred as heat rather than light.
Sankey
diagram for a filament lamp
Energy efficiency
You should
know that energy can be 'wasted' during energy transfers, and you should be
able to calculate theefficiency.
efficiency: The fraction of
the energy supplied to a device which is transferred in a useful form. of
a device.
'Wasted' energy
Energy
cannot be created or destroyed. It can only be transferred from one form to
another or moved. Energy that is 'wasted', like the heat energy from an
electric lamp, does not disappear. Instead, it is transferred into the
surroundings and spreads out so much that it becomes very difficult to do
anything useful with it.
Electric lamps
Ordinary
electric lamps contain a thin metal filament that glows when electricity passes
through it. However, most of the electrical energy is transferred as heat
energy instead of light energy. If you stroll down you will see the Sankey diagram for a typical
filament lamp.
Sankey
diagram for a filament lamp
Modern energy-saving lamps work in a different way. They
transfer a greater proportion of electrical energy as light energy. This is the
Sankey diagram for a typical energy-saving lamp.
Sankey diagram for a typical energy-saving lamp.
Source: http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011
Conclusive comparative statements:
From the Sankey diagram for a typical energy-saving lamp, you can see that much less electrical energy is transferred, or
'wasted', as heat energy in comparison with the energy loss in a filament lamp.The electrical energy is both cases is 100J. While the useful light energy given in the filament lamp is 10J, the useful light energy given in the energy-saving lamp is 75J. The waste energy in the filament lamp is the heat energy which is 90J while the waste energy thus the heat energy in the energy-saving lamp is 25J. The useful energy thus the light energy is much higher and the waste energy thus heat energy is much lower in the energy-saving lamp
Calculating efficiency
The efficiency of a device such as a lamp can be calculated
using this equation:
efficiency = ( useful energy transferred ÷ energy supplied ) ×
100
The efficiency of the filament lamp is (10 ÷ 100) × 100 = 10%.
This means that 10% of the electrical energy supplied is
transferred as light energy (90% is transferred as heat energy).
The efficiency of the energy-saving lamp is (75 ÷ 100) × 100 =
75%. This means that 75% of the electrical energy supplied is transferred as
light energy (25% is transferred as heat energy).
Note that
the efficiency of a device will always be less than 100%.
Sankey Diagram: Calculating Efficiency in an electric motor
Test: file:///C:/Users/User/Desktop/AQA-PHYS-W-SQP-1F.PDF
Questions Efficiency and Sankey diagrams
Efficiency (%) = (Useful energy produced/Total energy used) x 100
Q1. What is the efficiency of a wind up car that begins with 140 joules of elastic energy and produces 63 joules of kinetic energy?
Q2. What is the efficiency of a buzzer if it is supplied with 235 joules of electrical energy and produces 92 joules of sound energy?
Q3. What is the efficiency of a laptop that uses 12000 joules of energy and produces 3360 joules of light energy?
Now questions get a little trickier.
Q4. What is the efficiency of a light bulb that produces 8 joules of heat energy when it is supplied with 18 joules of electrical energy?
Q5. What is the efficiency of a yo-yo that begins with 84 joules of gravitational potential energy and produces 6 joules of heat energy as it falls?
Sankey diagrams - You need graph paper
Test: file:///C:/Users/User/Desktop/AQA-PHYS-W-SQP-1F.PDF
D raw a Sankey diagram to represent light bulb that uses 14 joules of electrical energy and produces 6 joules of light and 8 joules of heat. A mobile phone uses 40 joules of electrical energy and produces 25 joules of light and sound energy, while also producing 15 joules of heat.
Temperature
Metal takes in heat and emits energy
All objects take in and radiate heat (thermal) energy in the
form of infrared radiation. For example on a hot day a metal bench in the park
will absorb thermal energy and then it will give out (emit) thermal energy at
night as it cools down. A system(meaning anything where a change
can happen) must emit the same average power as it absorbs to be at a
constant temperature.
Different materials and
thermal energy
Some materials absorb and radiate thermal energy better than
others and this can be tested by comparing different surfaces to see which
heats up the fastest.
Thermal absorption in
surfaces
Surface
|
Absorbs
thermal energy
|
Emits
thermal energy
|
shiny
|
poor
|
poor
|
matt
|
good
|
good
|
light
|
poor
|
poor
|
dark
|
good
|
good
|
Energy and the future
Energy transfer and efficiency
Test
1. How
many forms of energy are there?
4
11
9
2. What is
another term for movement energy?
Kinetic energy
Change energy
Transfer
energy
3. If a
torch takes in 50J of energy and transfers 20J to light. What is its efficiency?
20%
50%
40%
4. A fan
takes in electrical energy and gives out movement, heat and sound. Which type
of energy is useful?
Heat
Movement
Sound
5. If 90J
of energy goes into a torch and 50J is given out as light, how much is given
out as heat - the only other product?
40J
90J
50J
6. Which
type of surface will absorb the most heat energy?
Shiny and light
Dark and matt
Shiny
and medium coloured
Answers:
9, kinetic,movement, 40J, dark and mat




Try this test now for convention
thttp://studylib.net/doc/6784198/study-island-assignment_thermal-energy-transfer
Answers: radiation, conduction, gases and liquids, radiation, convection, black, black, convection, head.
Conduction, convection and radiation
Source: http://www.bbc.co.uk/education/guides/zttrd2p/revision
Energy can be transferred by conduction, convection and radiation. Insulation is used to stop heat energy transfers from buildings and the human body.
Conduction
Heat is thermal energy. It can be transferred from one place to another by conduction.
Metals are good conductors of heat, but non-metals and gases are usually poor conductors. Poor conductors are called insulators.
Heat energy is conducted from the hot end of an object to the cold end.
Conduction in metals
The electrons in a piece of metal can leave their atoms and move about in the metal as free electrons. The parts of the metal atoms left behind are now positively charged metal ions.
The ions are packed closely together and they vibrate continually. The hotter the metal, the more kinetic energy these vibrations have. This kinetic energy is transferred from hot parts of the metal to cooler parts by the free electrons.
These move through the structure of the metal, colliding with ions as they go.
Investigating conductors
An experiment can be used to investigate which metal is the best conductor of heat. It involves some long thin strips of different metals (eg steel, aluminium and copper), wax, drawing pins and a Bunsen burner.
Method:
- Fix the drawing pin to the end of the metal strip using drops of wax.
- Position the other end of the metal strip into a Bunsen flame.
- Record the time taken for the wax to melt and the drawing pin to drop off.
The fastest time shows the best conductor of heat.
Variables that affect the time taken for the drawing pins to fall include the distance they are from the flame and the thickness of the metal.
If you have controlled all of these variables, you should find that copper conducts better than aluminium, while aluminium conducts better than steel.
Convection
Heat can be transferred from one place to another by convection.
Fluids
Liquids and gases are fluids because they can be made to flow. Theparticles in these fluids can move from place to place. Convection occurs when particles with a lot of heat energy in a liquid or gas move and take the place of particles with less heat energy.
Air current close to a radiator
Heat energy is transferred from hot places to cooler places by convection.

A beaker is heated and the coloured fluid inside shows convection currents
Liquids and gases expand when they are heated.
This is because the particles in liquids and gases move faster when they are heated than they do when they are cold.
As a result, the particles take up more volume. This is because the gap between particles widens, while the particles themselves stay the same size.
The liquid or gas in hot areas is less dense than the liquid or gas in cold areas, so it rises into the cold areas.
The denser cold liquid or gas falls into the warm areas.
In this way, convection currents that transfer heat from place to place are set up.
Convection currents can be seen in lava lamps. The wax inside the lamp warms up, becomes less dense than the liquid and so rises.
When it rises, it cools and becomes denser again, so it sinks. This same effect can be seen by putting a crystal of potassium permanganate in a beaker of water and gently heating it.
Convection explains why hot air balloons rise, and also why it is often hotter in the lofts of houses than downstairs.
As well as these examples, convection is seen on a much bigger scale in our weather and ocean currents.
Heat transfer by radiation
Heat can be transferred by infrared radiation. Unlike conductionand convection - which need particles - infrared radiation is a type ofelectromagnetic radiation that involves waves.

Light from the sun reaching earth
Because no particles are involved, radiation can even work through thevacuum of space. This is why we can still feel the heat of the Sun even though it is 150 million km away from the Earth.
Different surfaces
Some surfaces are better than others at reflecting and absorbinginfrared radiation. This table summarises some differences:
| Surface | Absorption | Emission |
|---|---|---|
| Dull, matt or rough | Good | Good |
| Shiny | Poor | Poor |
You can see that dull surfaces are good absorbers and emitters of infrared radiation. Shiny surfaces are poor absorbers and emitters (but they are good reflectors of infrared radiation).
If two objects made from the same material have identical volumes, a thin, flat object will radiate heat energy faster than a fat object.
This is one reason why domestic radiators are thin and flat.
Radiators are often painted with gloss paint, but they would be better at radiating heat if they were painted with matt paint instead. Also, despite their name, radiators actually transfer most of their heat to a room by convection, not radiation.
Heat radiation investigation
The transfer of infrared radiation from a hot object to cooler surroundings can be investigated using a piece of apparatus called Leslie’s cube.This is a metal cube with four side prepared in different ways: black, white, shiny, or dull. It can be filled with hot water or heated on an electrical hot plate so that all four sides are at the same temperature.
Method
- Measure the temperature a fixed distance from each side of a Leslie's cube using thermometers (or using a thermocouple, an electrical device that produces a potential difference depending on the temperature).
- Heat the Leslie’s cube with boiling water or with a hot plate.
- Continue to measure and record the temperatures every 30 seconds for five minutes, then plot a graph of temperature against time for each side.
- Compare the four graphs obtained. Remember that the four thermometers may vary in accuracy, so take this into account when analysing the results.
Reducing heat transfers – houses
Heat energy is lost from buildings through their roofs, windows, walls, floors and through gaps around windows and doors. However, there are ways that these losses can be reduced.
Heat escape routes
Take a look at this thermogram of a house. The roof and windows are the hottest, showing that most heat is lost from the house through those parts.

Thermogram of a house showing areas of heat loss
Heat energy is transferred from homes by conduction through the walls, floor, roof and windows.
It is also transferred from homes by convection. For example, cold air can enter the house through gaps in doors and windows, and convection currents can transfer heat energy in the loft to the roof tiles.
Heat energy also leaves the house by radiation - through the walls, roof and windows.
Ways to reduce heat loss
There are several different ways to reduce heat loss:
- Simple ways to reduce heat loss include fitting carpets, curtains and draught excluders. It is even possible to fit reflective foil in the walls or on them.
- Heat loss through windows can be reduced by using double glazing. These special windows have air or a vacuum between two panes of glass. If the double glazing has a vacuum there will be no conduction or convection. If the double glazing is made with air between the glass then convection is minimised because there is little room for the air to move. Air is a poor conductor so there will be very little heat loss by conduction.
- Heat loss through walls can be reduced using cavity wall insulation. This involves blowinginsulating material into the gap between the brick and the inside wall. Insulating materials are bad conductors and so this reduces the heat loss by conduction. The material also prevents air circulating inside the cavity, therefore reducing heat loss by convection.
- Heat loss through the roof can be reduced by laying loft insulation. This works in a similar way to cavity wall insulation.
Reducing heat transfers – the human body
Heat energy is lost from the human body. However, there are ways that these losses can be reduced.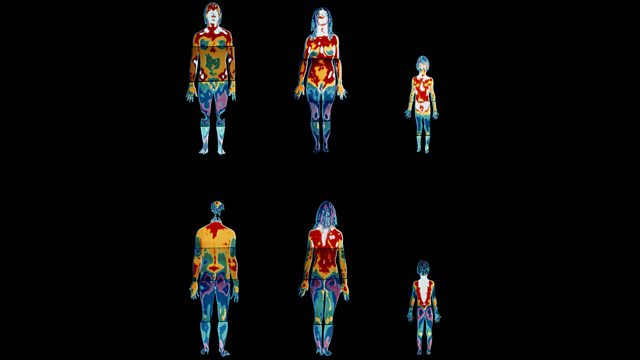 This is a thermogram of a man, woman and child not wearing clothes. The white and red parts are the hottest and so are losing most heat. Orange and green represent medium temperatures, and blue and purple are the coldest parts.It is easy to see that the heads of all three people are the hottest parts, followed by their torsos. Their arms and legs show medium temperatures, while their hands and feet are among the coldest parts. It is in these places that we sometimes get frostbite in very cold conditions.To reduce heat transfers from our bodies, we can wear insulatingclothing - such as gloves, scarves, hats and thermal socks - in cold weather. The more layers we wear, the more warm air can be trapped and the warmer we will remain. Some clothes, such as wool, are excellent insulators, because they trap lots of air.Glossary
This is a thermogram of a man, woman and child not wearing clothes. The white and red parts are the hottest and so are losing most heat. Orange and green represent medium temperatures, and blue and purple are the coldest parts.It is easy to see that the heads of all three people are the hottest parts, followed by their torsos. Their arms and legs show medium temperatures, while their hands and feet are among the coldest parts. It is in these places that we sometimes get frostbite in very cold conditions.To reduce heat transfers from our bodies, we can wear insulatingclothing - such as gloves, scarves, hats and thermal socks - in cold weather. The more layers we wear, the more warm air can be trapped and the warmer we will remain. Some clothes, such as wool, are excellent insulators, because they trap lots of air.Glossary- atomAll elements are made of atoms. An atom consists of a nucleus containing protons and neutrons, surrounded by electrons.
- conductionThe transfer of heat through a material by transferring kinetic energy from one particle to another.
- conductorA material which allows charge to move easily through it.
- convectionThe transfer of heat energy through a moving liquid or gas.
- denseCrowded closely together.
- electronSub-atomic particle, with a negative charge and a negligible mass relative to protons and neutrons.
- frostbiteDamage to the skin and other tissues that happens because of freezing. Severe frostbite can lead to loss of body parts such a fingers and toes.
- infrared radiationElectromagnetic radiation emitted from a hot object.
- insulateTo help maintain the temperature.
- ionElectrically charged particle, formed when an atom or molecule gains or loses electrons.
- kinetic energyThe energy that moving objects have.
- particleA general term for a small piece of matter. It can mean protons, neutrons, electrons, atoms, ions or molecules for example.
- radiationEnergy carried by particles from a radioactive substance, or spreading out from a source.
- thermogramImage made by detecting infrared radiation rather than visible light. Different colours are used to represent the different temperatures of the objects in the image.
- vacuumA volume that contains no matter.
Try this test now for convention
thttp://studylib.net/doc/6784198/study-island-assignment_thermal-energy-transfer
- Another test on Conduction, convection and radiation
1
Which of these methods of heat transfer does not involve particles?
2
What method of heat transfer occurs mainly in solids?
3
Which states of matter are fluids?
4
What method of heat transfer can occur in a vacuum?
5
What method of heat transfer occurs when particles with a lot of energy take the place of those with less?
6
What method of heat transfer do we see in our weather patterns?
7
What surfaces best absorb infrared radiation?
8
What surfaces best emit infrared radiation?
9
What method of heat transfer explains why hot air balloons rise?
10
Which part of the human body loses the most heat?
Answers: radiation, conduction, gases and liquids, radiation, convection, black, black, convection, head.













REVISE AND SUCCEED IN PHYSICS GCSE!
ReplyDeletetruly helpful
ReplyDelete